The hope of children's immunotherapy
About the Centre
The Ian Frazer Centre for Children’s Immunotherapy Research is an innovative research centre looking to accelerate children’s cancer breakthroughs and hasten the delivery of better treatments to patients. Under the guidance of Professor Ian Frazer – a pioneering cancer researcher, world leading immunotherapy expert, and credited with co-inventing Gardasil, the world’s first vaccine for cancer – the Centre is the first dedicated to children’s immunotherapy research in the country.
To date, children have not benefited from all advances in cancer immunotherapy to the same extent as adults. This state-of-the-art Centre will conduct desperately needed research to revolutionise the ability to treat children with cancer using this approach.
Cancer kills more children than any other disease, and while survival rates have improved, children still die from cancer. Now is time to address the gap.
Bringing together researchers and clinicians with expertise across the full translational research pathway, the Centre will see treatments that are being developed in labs move through to application in clinical practice.

Professor Ian Frazer AC
Professor Ian Frazer is a pioneering cancer researcher, world-leading immunotherapy expert, and credited with coinventing Gardasil™, the world’s first vaccine for cancer.
Foundation President of the Australian Academy of Health and Medical Sciences, Chair of the Advisory Board for the Medical Research Future Fund, Australian of the Year in 2006, Recipient of the Prime Ministers Prize for Science, Awarded the Balzan Prize, in 2008, Fellow of the Royal Society of London and appointed Companion of the Order of Australia in 2013.

The Centre aims to find new ways to harness immunotherapy, and ensure current clinical research is translated into a new era of children’s cancer treatments.
Professor Ian Frazer AC
Professor Di Yu
Professor Di Yu has been appointed as the Centre Director, using his expert knowledge of immunotherapy to lead major research programs and guide various collaborations for clinical translation. Professor Yu is the inaugural Chair of Paediatric Immunotherapy within the Child Health Research Centre of the University of Queensland and a Professor of Immunology at the University of Queensland Frazer Institute.
He received his PhD from Australian National University in 2007 and postdoctoral training at the Garvan Institute of Medical Research from 2008-2010. Before joining the University of Queensland, he was a faculty member at Monash University from 2011-2016 and at Australian National University from 2017-2019.
Professor Yu is a Fellow of the Australian Academy of Health and Medical Sciences and was awarded the prestigious Jian Zhou Medal by the Academy in 2021, in recognition of his landmark discoveries in revealing the differentiation and functions of T cells in human health and disease, research which has enabled new diagnosis and therapy for autoimmune, allergic, and infectious diseases, and the improvement for vaccine efficacy.

Research
What is the Research for?
The Centre will conduct preclinical and translational research to:
* Discover and characterise new targets for immunotherapies
* Design models to test the effectiveness of paediatric immunotherapies
* Develop new treatments and treatment regimens
* Monitor the effectiveness of existing immunotherapies to predict treatment response
* Study the mechanism of action of immunotherapy alone or in combination with other therapies
* Improve the understanding of tumour immunity in paediatric cancer patients
PROJECT 1
PiggyBac transposon UCB-CAR19-NK cells: a novel off-the-shelf cellular immunotherapy for children with CD19+ blood cancers.
Professor Maher Gandhi, The University of Queensland
Challenge
Chimeric Antigen Receptor (CAR) T cell immunotherapies have resulted in unprecedented responses in relapsed/refractory acute lymphoblastic leukemia and chronic lymphocytic leukemia. But cost and State-based inequality of access prevent their revolutionary potential from being realised. Specifically, three problems remain: 1) the delay in manufacture means that many children will die before the immunotherapy is generated – an off-the-shelf product is needed; 2) side-effects (cytokine release syndrome) which can be devastating; 3) Application to other blood cancer and solid tumours.
Opportunity
This study aims to lay the foundations to generate an affordable, effective and safe off-the-shelf CAR cellular immunotherapy, to produce a ready-to-go treatment for acute lymphoblastic leukemia and chronic lymphocytic leukemia, that can be safely infused into patients without graft versus host disease.
Outcome
This research is an important step towards allowing future generations of cancer patients to benefit from CAR cellular immunotherapies and will tackle problems 1-3 above. The future plan is to extend this technology to other types of cancer including other blood cancers and solid tumours.
PROJECT 2
Novel human preclinical tools to better predict outcomes for immunotherapy.
Associate Professor Kristen Radford, The University of Queensland
Challenge
Over 230 Australian children are diagnosed with leukemia each year. They receive intensive chemotherapy which can have long term side-effects and some have poor chances of survival. Immunotherapies have enormous potential for the treatment of paediatric cancers, especially those for which current treatments are ineffective or associated with toxicity and/or high risk of relapse.
Opportunity
New treatments, which use the patient’s immune system, are desperately needed and hold promise of improving survival whilst reducing treatment-related toxicity for leukemia patients. Preclinical studies are designed to test the safety and effectiveness of new treatments before testing in humans and primarily use animal models. However, there are no reliable preclinical models that can evaluate new human-specific immunotherapies and prioritise those most likely to be successful for clinical trial.
Outcome
This project aims to establish a new model that will help researchers test how the human immune system responds to a potential treatment before reaching humans. The model will be comprised of human leukemia and a patient matched human immune system that can more effectively identify the best new immunotherapies to trial in childhood leukemias. The researchers have developed a new vaccine they would like to test before proceeding to clinical trial.
PROJECT 3
Targeting and eliminating paediatric cancers with chimeric antigen receptor engineered natural killer cells, a new hope for cancer immunotherapy.
Dr Fernando Guimaraes, The University of Queensland
Challenge
In Queensland, brain cancers are the leading cause of disease-related death in children, and bone cancers in adolescents. Medulloblastoma and osteosarcoma are the most common malignant brain and bone tumours, respectively, both with marginal survival chances when these tumours are refractory or recurrent. Current cancer immunotherapies largely focus on genetic engineering of T cells, a type of white cells, taken from the patient’s own blood. However, such approaches suffer from significant negative side effects and technical challenges related to the need to build the T cells from each patient, specifically.
Opportunity
Natural killer (NK) cells are a type of white cells known for their ability to kill cancer cells. NK cells are very promising as cancer treatment as they do not attack healthy tissues and are less toxic than conventional therapies. To address the unmet need for effective, widely and rapidly accessible immunotherapies, the research team will employ genetic engineering techniques to generate NK cells that can kill cancer cells more efficiently and be used in any patient. This project will investigate the efficacy of these cells in these two types of cancers – medulloblastoma and osteosarcoma.
Outcome
The vision of this project is to create “super-killer” NK cells to deliver a new, safe, and highly effective cancer treatment, with the aim to develop a clinic-ready super-killer NK product for generic off-the-shelf use against solid cancers. This project will have a significant health and economic impact and will attract investments and commercial opportunities to further develop the technology to treat this devastating disease in children.
The Ian Frazer Centre for Children’s Immunotherapy Research is led by The University of Queensland in collaboration with Children’s Health Queensland and QIMR Berghofer Medical Research Institute.
Charlotte's story
Charlotte was four months shy of her fourteenth birthday when she passed away from Ewing Sarcoma. She had planned on going to uni, helping others, travelling, and having a “cute husband” (in the words of Isabel, her best friend).
Charlotte had bravely endured more than five years of cancer treatment, including eight invasive surgeries, seven conventional cancer treatments, two traditional radiation treatments, and two experimental radiation treatments. But she died in her parent’s arms before she could undergo targeted immunotherapy, because it wasn’t ready yet. It wasn’t ready when she needed it most.
Charlotte didn’t have the chance to benefit from the progressive treatment that has saved so many adult lives, and it’s because of this that the Ian Frazer Centre is so important. The Centre aims to ensure that all children facing a cancer diagnosis have the opportunity for a more effective cancer treatment, and that they will recover and live long, healthy, and happy lives. That will be Charlotte’s legacy.
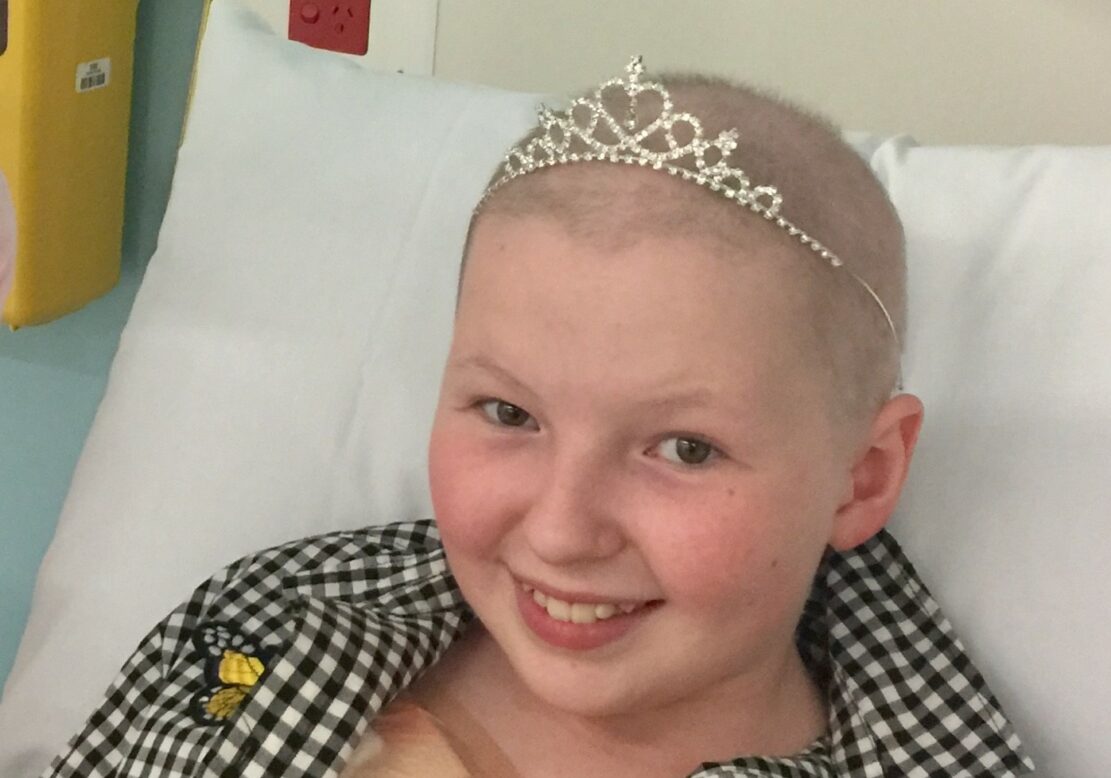
Our wish is that all children with cancer have the opportunity for a less aggressive and more effective treatment.
Michelle and Michael, Charlotte’s Parents
The facts
Make a significant gift
If you would like to give a gift over $1000, our team would love to speak to you.
By giving, you will join a special group of visionary leaders in business and philanthropy, attached to the opportunity to be there at the beginning, to stand with Professor Frazer, as we work toward historic medical breakthroughs.
Together, we can help make immunotherapy treatments more readily available for children as the Centre progresses.
For a confidential discussion, please reach out to our Philanthropy Team using the contact form or call Nadine Moore, Director of Philanthropy and Government Relations on 07 3606 6177.
"*" indicates required fields


